


MRI-Guided Focused Ultrasound System Passes Special Review by National Medical Devices Agency
June 15, 2024
The National Medical Devices Agency has announced that the MRI-guided phased array focused ultrasound technology, also known as "Magnetic Knife" technology, developed by the team of Academician Chen Yazhu and Researcher Shen Guofeng from Shanghai Jiao Tong University's School of Biomedical Engineering and the National Engineering Research Center for High-End Technology of MRI Diagnosis and Treatment, has passed the special review for innovative medical devices.

The Magnetic Knife technology is an emerging treatment method for various serious diseases, including benign and malignant tumors, cardiovascular and neurological diseases. It operates under real-time MRI imaging guidance to focus ultrasonic beams emitted from hundreds to thousands of ultrasonic elements onto the body's lesion site, producing thermal ablation and other biological effects. The system combines real-time MRI temperature measurement and closed-loop control of ultrasonic energy output to accurately treat targets in the body and brain.
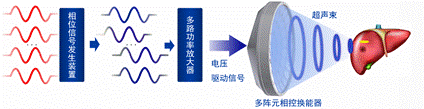
The team has made significant breakthroughs in core technologies, including the development of a multi-element ultrasonic rapid phase calibration compensation algorithm and a high-speed focus scanning method. These innovations address challenges such as ultrasonic penetration through the skull, beam refraction, and rib obstruction, enabling the system to navigate around the ribs and penetrate the skull for multimodal treatment. Furthermore, the team has proposed a precise mapping method for the coordinates of MRI and phased focused ultrasound systems, as well as a method for rapid and large-range precise temperature measurement and closed-loop temperature feedback control of ultrasonic dosage output. This ensures precise lesion targeting and accurate thermal dose delivery based on non-destructive temperature measurement and feedback monitoring, enhancing the safety and effectiveness of the treatment.
The product that passed the special review is a body Magnetic Knife product for the treatment of uterine fibroids and has completed all clinical trials, with the application for a Class III medical device registration certificate underway. Products for the treatment of adenomyosis and bone tumors are also nearing completion of clinical trials and will soon apply for the same registration. The team, under the guidance of Academician Chen Yazhu, has been conducting interdisciplinary cooperation with more than 40 renowned hospitals and medical technology companies, focusing on cutting-edge research and clinical work for the Magnetic Knife. They have overcome key core technologies that were previously bottlenecks.

The team's strategy of "1+N+X" is based on the Magnetic Knife technology platform, targeting various medical disciplines and conditions, including gynecology, orthopedics, urology, breast surgery, neurosurgery, and neurology, with a focus on non-invasive treatment for conditions such as uterine fibroids, bone tumors, and neurological and mental diseases.
Recently, the team's head Magnetic Knife system successfully completed preclinical experiments on primates for the first time in China, providing crucial practical support for the system's entry into human clinical trials and its domestication. The first generation of the domestic Magnetic Knife system for brain disease treatment has passed ethical review by Beijing Xuanwu Hospital and will soon conduct the first clinical trial for tremor-dominant Parkinson's disease. The product, upon market launch, will focus on thermal ablation treatment for essential tremor, tremor-dominant Parkinson's disease, and movement disorder-type Parkinson's disease, with plans to expand to other innovative treatments for brain tumors, epilepsy, Alzheimer's disease, ALS, neuropathic pain, depression, and obsessive-compulsive disorder, showcasing a broad application prospect.