


Weiliang Xia’s team from School of Biomedical Engineering, Shanghai Jiaotong University Published Oncogene and Theranostics Papers: The Discovery of a New Mechanism of Fibroblast Growth Factor Signaling Pathway in Lung Cancer Regulation
March 15, 2020
The largest growth factor family, fibroblast growth factors (FGFs) family has 22 members. FGFs in tissues mediate metabolic function, tissue repair and regeneration by reactivating development signaling pathways, and participate in maintaining a variety of key biological functions, including cell growth, differentiation, angiogenesis, embryo development, wound healing and repair, and cell metabolic regulation.
The amplification frequency of FGF19, FGF3, FGF4 and CCND1 in smoking lung squamous cell carcinoma (S-LSCC) was 5 times higher than that of non-smoking lung squamous cell carcinoma (NS-LSCC). FGF19 amplification frequency was verified in independent LSCC clinical samples. Furthermore, FGF19 can promote LSCC cell proliferation in vitro. These data above suggests that FGF19 is a potential driver gene in smoking patients with LSCC.
On this basis, Xia’s team from School of Biomedical Engineering, Shanghai Jiaotong University continued to explore the potential mechanism of LSCC progression by driving gene FGF19 and its main receptor FGFR4. Researchers have found that smoking or chemotherapy can cause endoplasmic reticulum stress, and further upregulated FGF19 in lung cancer cells. For LSCC with high FGF19 expression, Xia’s team proved it possible using AZD2014 to inhibit mTOR pathway in tumor, providing a new strategy for direct mTOR pathway-based or targeted inhibition of FGF19/FGFR4 therapy in FGF19-driven LSCC. The paper was published in nature oncology journal, Oncogene, on February 28, 2020.

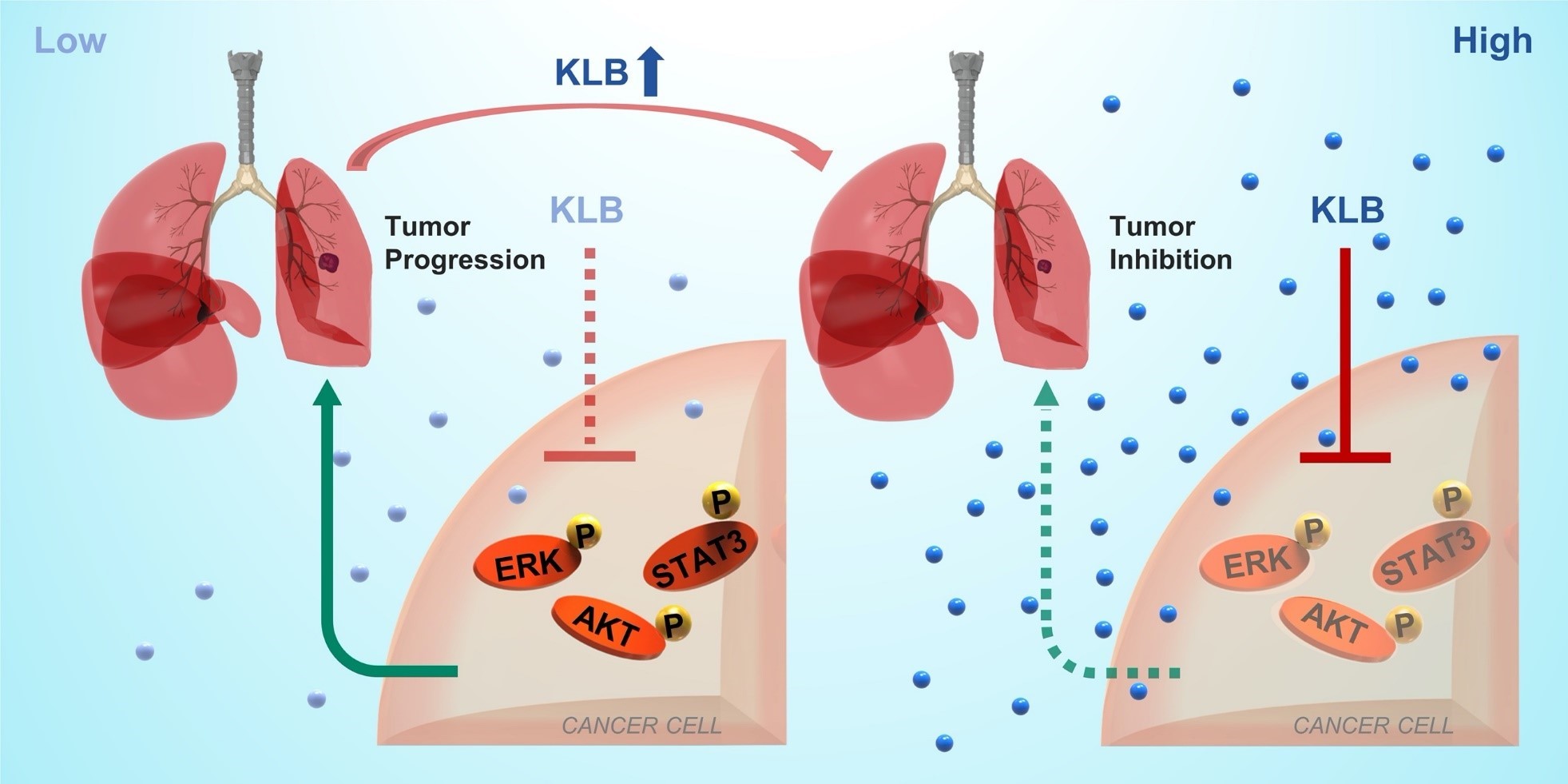
The receptor binding process of FGF19, an endocrine growth factor, requires help from KLB. KLB (βKlotho) from Klotho family can form FGF19-KLB-FGFR4 complex, activate a variety of downstream signaling pathways, affect angiogenesis, cell differentiation and other biological processes. β-Klotho belongs to Klotho family, playing an important role in cell energy metabolism, aging and muscle regeneration. Because α-Klotho (also from Klotho family) overexpression can significantly prolong mice’s life span, it is universally recognized as an anti-aging gene.
In addition, studies have shown that α-Klotho’s promoter region is prone to hypermethylation, leading to expression decrease in cancer tissues. α-Klotho overexpression can inhibit cancer oncogenesis and development of many kinds. Though β- and α-Klotho share 41% similarity in amino acid sequence, the mechanism of FGF19 co-receptor β-Klotho remains unclear in non-small cell lung cancer (NSCLC).
Thanks to large numbers of clinical samples, Xia’s team compared the β-Klotho expression in tumor and adjacent non-tumor tissues of NSCLC, as well as the β-Klotho level in the serum of tumor patients and normal people. The clinical significance of β-Klotho as a diagnostic/prognostic biomarker was discussed. The study found that β-Klotho expression is low in tumor tissues, which can aid NSCLC diagnosis.
This study also tested how β-Klotho affects tumor bearing mice in vivo and in vitro. Using various murine models (in situ model and subcutaneous tumor model) and cell experiments, the researchers found that exogenous β-Klotho administration or overexpression can promote cell apoptosis and cell cycle arrest, along with the inactivation of ERK1/2, Akt and STAT3 signal, and inhibit the proliferation and metastasis of NSCLC. This shows that β- Klotho has potential diagnostic/therapeutic significance in NSCLC. In addition, after referring to many databases, it was found that β-Klotho expression was negatively correlated with lymph node metastasis, overall survival and progression free survival rate. Xia’s team believes that β Klotho has the potential to be a new target for NSCLC treatment, and clinical application in the future is expected. Relevant findings of β-Klotho have been published in Theranostics, a world-renowned diagnosis and treatment journal in October 2019.
These two projects are supported by the general fund of National Natural Science Foundation of China and Med-X key general projects of Shanghai Jiaotong University. Professor Xia is the corresponding author, doctoral student Fan Li as first author. It’s the 10th paper this research series produces since Xia's team first published cooperative paper (2013) in lung cancer with Shun Lu's team, director of thoracic Hospital Affiliated to Shanghai Jiaotong University.
They have published 6 joint communication papers in Oncogene, Cancer Letters and other oncology journals. The main findings are (1) the molecular mechanism of FGFR1 regulating LSCC stem cells phenotype through Hedgehog signaling pathway, and proposed the combination treatment of FGFR1 and GLI2 inhibitor in Hedgehog for LSCC (Oncotarget, 2016). (2) FGFR1 interacts with Hippo/Yap signaling pathway to regulate lung cancer metastasis and dry phenotype, which explains the theoretical basis of clinical application of FGFR1 as a diagnostic marker (Cancer Lett, 2018) (3) FGFR signal is also involved in regulating lung cancer cells autophagy (J exp Clin Cancer Res, 2017) and EGER1-ERK1/2-SOX2 signal axis in epithelial mesenchymal transition and metastasis of lung cancer cells (Oncogene, 2018). These work reveals the complexity of FGFs-FGFRs signal regulation and the basis of clinical application of simultaneous intervention of downstream targets. Recent work is based on further study of FGF/FGFR signal axis’s role in lung cancer.
Full text links:
1. https://www.nature.com/articles/s41388-020-1227-2