


Li Li's team reveals how histone methylase SETD2 cooperates with Kras to regulate the occurrence and development of pancreatic cancer
July 29, 2019
Li’s team, School of Biomedical and Engineering, Shanghai Jiao Tong University, together with Jing Xue’s team from Renji Hospital, published their new research “Loss of Setd2 promotes Kras-induced acinar-to-ductal metaplasia and epithelia–mesenchymal transition during pancreatic carcinogenesis” on GUT on July 11, 2019.
They explained the SETD2 depletion synergistic with KRAS in the development of pancreatic cancer and mechanisms of SETD2 in the initiation and metastasis of pancreatic cancer.
Their findings highlight the function of SETD2 during pancreatic carcinogenesis, which make better understanding of epigenetic dysregulation in PDAC. Moreover, it may also pave the way for development of targeted, patients-tailored therapies for PDAC patients with SETD2 deficiency.
Pancreatic cancer is known as the "king of cancer", which has a high degree of malignancy, rapid development and deterioration, and most of it is hard to cure when diagnosed. Up to now, there is no effective treatment method. Therefore, the research on its pathogenesis is extremely urgent. More than 90% of pancreatic cancers are produced by exocrine cells, which is called Pancreatic ductal adenocarcinoma (PDAC). Initiation and metastasis are the two most important stages in the occurrence and development of PDAC. Both inflammation and Kras mutation can lead to acinar duct metaplasia (ADM), and it progresses irreversibly when Kras is continuously activated. At present, ADM is considered to be an important event of PDAC initiation. In this case of tumor suppressor gene imbalance or inflammation, toxicity and other stimuli, the duct-like cells of precancerous lesions will further progress to PDAC. Pancreatic tumors are prone to distant metastasis, which is the main cause of death in patients with pancreatic cancer. Epithelial-Mesenchymal Transdifferentiation (EMT) is the first event of cancer cell metastasis. Abnormal appearance regulation is one of the important characteristics of malignant tumors. In recent years, it has been found that a large number of appearance modification abnormalities also exist in pancreatic tumors, but the related mechanism and function research still remain to be disclosed.
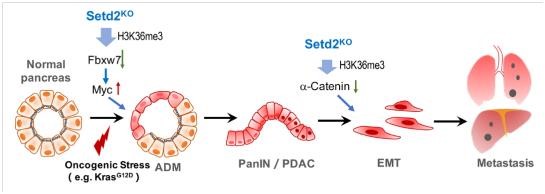
Associate researcher Li Li established the Setd2 conditional knockout mouse model in 2014 and carried out extensive functional research in the fields of reproduction, cultivation and cancer treatment (Zuo et al, J Bio Chem, 2018; Wang et al, PLoS Biol, 2018; Xu et al, Nat Genet, 2019). The cooperative group carried out further research, combined with the shared database and clinical samples, they found that the expression of SETD2 and H3K36me3 was significantly negatively correlated with the prognosis of patients with pancreatic cancer. By constructing pancreas-specific knockout Setd2 mice and combining with Kras mutations, SETD2 deletion can greatly promote the process of pancreatic cancer induced by KRAS. High-throughput expression profiles and H3K36me3 ChIP analysis were performed on acinar cells and pancreatic cancer cells with Setd2 wild-type and Setd2 missing samples. Setd2 played a role by regulating different downstream genes in the two pathological stages: 1) Initiation Stage: Re gulates the expression of E3 ubiquitin ligase Fbxw7 of MYC protein, which affects the homeostasis of acinar cells, thereby promoting the occurrence of ADM; 2) Metastasis stage: Regulates the expression of Ctnna1 adhesion protein, affects the stability of E-cadherin, and promotes EMT and metastasis. Associate researcher Ningning Niu and Dr. Lu Ping are the co-first authors of the paper; Researcher Jing Xue, Associate Researcher Li Li and Director Yongwei Sun are the co-corresponding authors of this paper.
Article link:http://dx.doi.org/10.1136/gutjnl-2019-318362