


New discovery from Weiqiang Gao and Li Li’s group: BRG1 attenuates colonic inflammation and tumorigenesis through autophagy-dependent oxidative stress sequestration
October 11, 2019
On October 10, 2019, Weiqiang Gao and Li Li’s group from the School of Biomedical Engineering, Shanghai Jiao Tong University, published their newly research “BRG1 attenuates colonic inflammation and tumorigenesis through autophagy-dependent oxidative stress sequestration” on Nature Communications online. They found the important role of BRG1 in colon inflammation and tumor genesis and development, and clarified its mechanism in maintaining intestinal
Autophagy is a central component of integrated stress responses that influences many inflammatory diseases, including inflammatory bowel disease (IBD) and colorectal cancer (CRC). While the core machinery is known, the molecular basis of the epigenetic regulation of autophagy and its role in colon inflammation remain largely undefined. Here, they report that BRG1, an ATPase subunit of the SWI/SNF chromatin remodeling complex, is required for the homeostatic maintenance of intestinal epithelial cells (IECs) to prevent the inflammation and tumorigenesis. BRG1 emerges as a key regulator that directly governs the transcription of Atg16l1, Ambra1, Atg7 and Wipi2, which are important for autophagosome biogenesis. Defective autophagy in BRG1-deficient IECs results in excess reactive oxygen species (ROS), which leads to the defects in barrier integrity. Their results establish that BRG1 may represent an autophagy checkpoint that is pathogenetically linked to colitis and is therefore likely a potential therapeutic target for disease intervention.
Tissue inflammation inevitably results in tissue repair and tumorigenesis. Patients with inflammatory bowel disease (IBD), such as Crohn’s disease (CD) and ulcerative colitis (UC), exhibit a higher risk of developing colorectal cancer (CRC). IBD results from a complex series of interactions between the mucosal barrier, the environment, and the immune system. Barrier integrity is essential for preventing invasion of microorganisms and development of chronic inflammations. Thus, the defects in maintaining the homeostasis of intestinal epithelial cells (IECs) are known to trigger chronic inflammation and repair, which occasionally results in dysplasia.
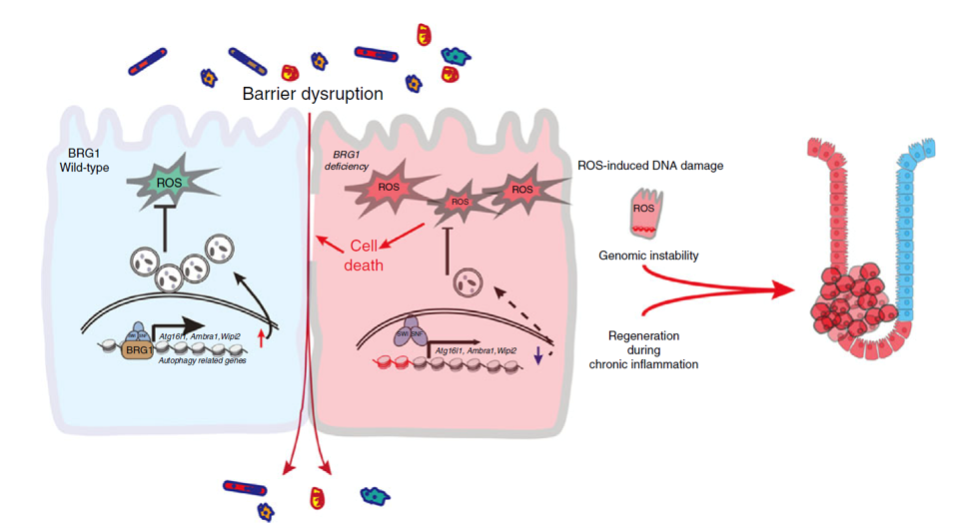
Their results highlight the importance that BRG1 functions as a homeostatic regulator that integrates the intact mucosal barrier by modulating autophagy-dependent oxidative stress in the colon (Fig. 7i). Inflammation is closely associated with tumorigenesis, and IBD is a predisposing factor for CRC. Thus, our results establish a role for BRG1 in inflammation-associated CRC and may provide insight into understanding of other human disorders associated with BRG1 deficiency.
Min Liu, a doctoral student, the School of Biomedical Engineering, Shanghai Jiaotong University, and Tongyu Sun, doctoral student in the Institute of Nutrition and Health, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, are the co-first authors of the paper. Professor Weiqiang Gao, the School of Biomedical Engineering, Shanghai Jiaotong University and associate researcher Li Li are the co-corresponding authors. This research was supported by the National Key Research and Development Program of the Ministry of Science and Technology, the National Natural Science Foundation of China, the Shanghai Municipal Science and Technology Commission.
Article Link: https://www.nature.com/articles/s41467-019-12573-z